Synthetic Biology Spotlight: Amyris
By Chris Calvey, postdoctoral researcher in microbial metabolic engineering at the National Renewable Energy Lab (NREL)
Special to The Digest
This post is the first of a series intended to take a closer look at the science behind some of the most promising biotech companies in the renewable energy sector.
First of all, what is synthetic biology exactly?
Simply put: synthetic biology is a revolutionary new field of science which involves genetically modifying lifeforms – usually simple ones like bacteria or yeast – and redesigning them to do something interesting or useful for us humans. In practice, this typically involves both removing some genes (which are not needed for the task at hand) and introducing new genes that are borrowed from other species. We call this tinkering process of adding and deleting genes “metabolic engineering.”
The great promise of synthetic biology is that by carefully tweaking the metabolism of microbes, we can convert them into customizable mini-factories for making almost anything we want. As you’ll see, the biotech industry is harnessing the power of synthetic biology to create a cornucopia of new foods, fuels, materials, medicines, and a whole lot more that can improve the world.
Best of all, these bio-based alternatives are sourced from renewable inputs and almost always have a lower carbon footprint than the products they replace. Which means synthetic biologists and their billions of microbial minions are helping to create a more sustainable future for this planet! One of the great pioneers in the world of synthetic biology is a company called Amyris.
Amyris [NASDAQ: AMRS]

Synthetic Biology: Putting yeast to work for you (Source: Amyris)
What’s the Amyris origin story?
Amyris is old-school cool. Back in the 2000s, they had the brilliant idea of using metabolic engineering to design a new yeast capable of turning table sugar (sourced from renewable sugarcane) into gasoline. And it worked! Their replacement was a hydrocarbon called farnesene, and they made tons of it at their giant biorefinery in Brazil.
But the problem was… oil is actually really cheap, which makes it really really hard to compete against. Amyris’s fuel is undoubtedly cleaner and greener than gasoline, but it ain’t cheaper… and capitalism says that’s all that matters. So Amyris did something really smart.
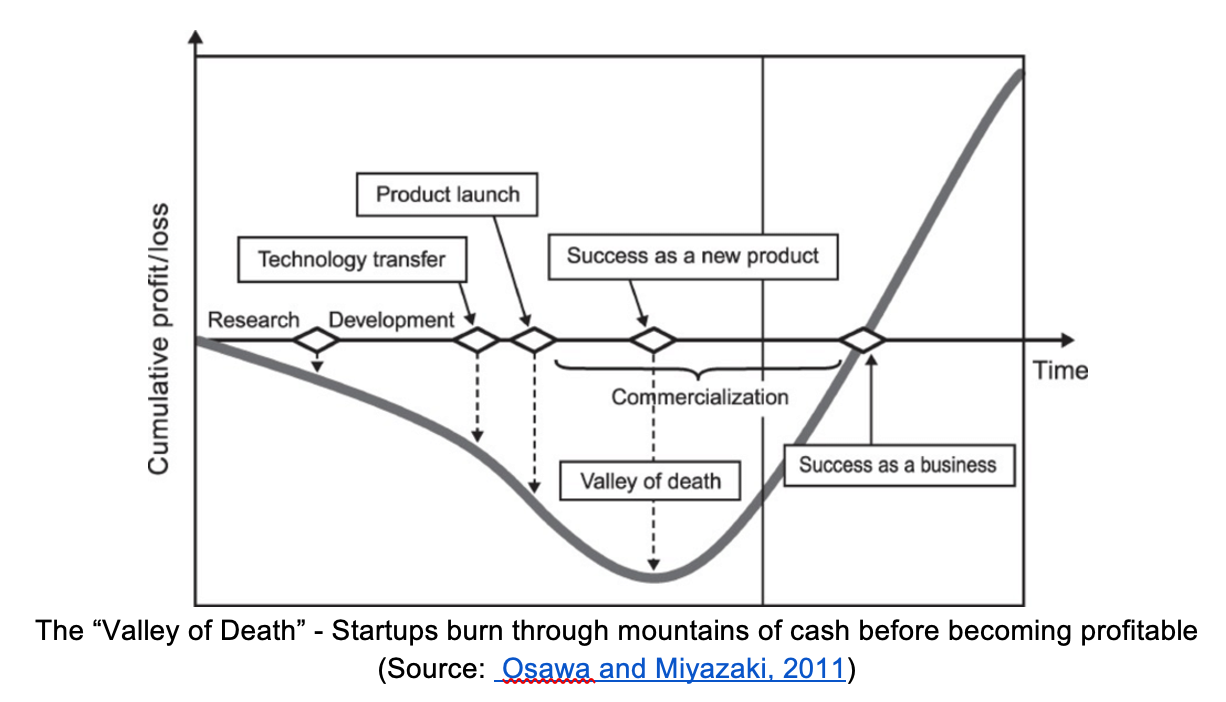
They paused that effort and pivoted to more lucrative markets, like complex flavors and fragrances. The idea here is that it’s better to make a small amount of something which you can sell for a lot of money, than it is to make loads of a relatively low-value product like gasoline. This pivot allowed Amyris to survive the dreaded “valley of death” that consumes so many biotech companies in their infancy.
The “Valley of Death” – Startups burn through mountains of cash before becoming profitable
(Source: Osawa and Miyazaki, 2011)
What does Amyris do best?
In theory, almost any biomolecule could be manufactured in yeast by simply introducing the appropriate biochemical pathways – consisting of a dozen or so steps carried out by a series of genes. But in nature, there are likely many thousands of slightly different versions of each gene to choose from, and it’s hard to know which one will work best. As you can imagine, testing the exponentially growing number of possible gene combinations (and fine-tuning their expression levels) quickly becomes very daunting. Luckily, Amyris has mastered the art of strain construction.
Amyris has a veritable army of robots at their disposal. Specifically, the super nerdy kind that is really good at stitching together DNA and rapidly cranking out new yeast strains. Those robots can then pass along the engineered yeasts to yet more robots to evaluate their performance. These skills come in handy whenever Amyris decides they want to develop a new product.
Their automated robotic systems allow them to screen huge numbers of new yeast strains very quickly. The slacker strains get sent to the dumpster, while the best performing strains get selected for even more rounds of engineering and optimization. In the synthetic biology world, we call this the “DBTL” cycle: design, build, test, and learn. Ultimately this means Amyris can enter the market with a new product in a matter of months or years, instead of decades.

Robots like this colony-picker greatly speed up strain construction (Source: SciRobotics)
Amyris has used their technology platform to make a ridiculously wide variety of products. And remember, all of this comes from engineered yeasts fermenting sugarcane juice
- An anti-malarial drug called artemisinin.
- Vitamin E for use in human nutrition and animal feed.
- Specialty rare oncology drugs for treating cancer patients.
- Low carbon animal and fermentation-based protein production.
- A low-calorie sweetener (RebM) – basically making stevia without the plant.
- Highly purified cannabinoids like cannabigerol – basically CBD without the weed.
- Fragrances copied from plants like sage, sandalwood, patchouli, and rare pine trees.
- A huge range of squalane-containing products in the clean beauty and skincare worlds.
Amryis’s foray into the beauty world has been especially successful. Their “Pipette” (baby/mother focused) and “Biossance” (adult focused) cosmetic brands are among the fastest growing in the skincare industry. You can find Amyris products in real life brick and mortar stores like Sephora and Target – a rare feat in the emerging bioeconomy! Biossance has even scored some big-time celebrity endorsements from actress Reese Witherspoon and grooming extraordinaire Jonathan Van Ness. Eagle-eyed digesterati may have even spotted Van Ness showing off some Biossance products in the latest season of Netflix’s Queer Eye.
Closing Thoughts
The Amyris Biossance product line centers around a molecule called squalane, prized for its moisturizing and antioxidant properties as a skin emollient. Until recently, the primary naturally occurring source of squalane used by the cosmetics industry was derived from shark liver oil. It’s estimated that millions of deep-sea sharks are still harvested every year for their meat, fins, and squalene-rich livers. That’s what makes Amyris such a compelling success story. They’ve proven that synthetic biology can mimic mother nature to make almost anything, and help to spare the lives of innumerable endangered plants and animals that would otherwise be consumed by humans for their useful properties.
So there you have it. Amyris: the story of the yeast engineering company that went from making gasoline to making sunscreen, and earned it’s crown as one of the most promising publicly traded synthetic biology companies.
About the Author
Chris Calvey is a postdoctoral researcher in microbial metabolic engineering at the National Renewable Energy Lab. He was also a member of the team that created the world’s first “synthetic” bacterial cell. In his spare time, he enjoys writing about the intersections of science, politics, and renewable energy at chriscalvey.com.
Category: Top Stories, Thought Leadership















