Badass POP missile fuel arrives from Berkeley’s labs: Will America keep a hold of them?

News has arrived out of Berkeley of an advanced biofuel so powerful and transformative that we suspect it may never reach consumer markets. These are POP fuels, and you can’t have them.
Simulation data from Sandia suggests “that POP fuel candidates are safe and stable at room temperature and will have energy density values of more than 50 megajoules per liter after chemical processing.
That’s 56 percent more energy than gasoline, per gallon. 43 percent better than kerosene.
And there’s the rub. No one begrudges the consumer a shot at a POP fuel that would provide as much as 56 percent more range per fill up. That’s a lot of help for pain at the pump.
But….there’s a big but.
This fuel is even 25 percent more energy-dense than J-10, used for missile fuel and costing the military something like $25 per gallon because that thrust is so highly valued.
You Will Never See this Fuel at the Service Station – Here’s Why
Who’s up for some eco-friendly space travel? asks Berkeley Lab’s Aliyah Kovner. It conjures up a sweet vision of low-carbon transit to Mars. Worth noting that Mars if the God of War, and for sure the military will gobble up all the fuel ever produced via this pathway for a long, long time.
Here’s the need to know, in practical terms. North Korea’s Hwasong-14 missile has a potential range of 8,000km, according to this BBC report.
A 25 percent range boost takes the Hwasong-14 to 10,000 kilometers. Let’s visualize that with this chart.
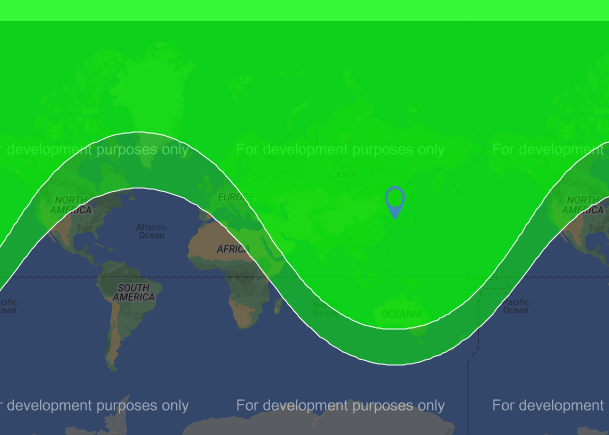
The bright green indicates the current range, and the dark green indicates the potential range. And if you’ve noticed that filling a Hwasong-14 missile with a POP fuel gives it a range to hit almost any target in the eastern two-thirds of the United States and most of Europe. According to the BBC, some studies have concluded that the Hwasong-14 could reach a range of 12,500 kilometers with maximum trajectory and POP fuels, which brings all of the US and Europe into range. And that, in a nutshell, is the primary reason you may never see a POP fuel anywhere near a commodity market, ever. This class of biofuels screams “classified”.
Why don’t US and other countries simply make these molecules from petroleum?
Um, you can’t, actually, by any known process or even dreamed-of process.
ABPDU researcher Eric Sundstrom, co-author of the paper, notes: “With petrochemical fuels, you get kind of a soup of different molecules and you don’t have a lot of fine control over those chemical structures. But that’s what we used for a long time and we designed all of our engines to run on petroleum derivatives,” said , an author on the paper describing POP fuel candidates published in the journal Joule, and a research scientist at Berkeley Lab’s Advanced Biofuels and Bioproducts Process Development Unit (ABPDU).
What are they?
In a nutshell, they are polycylcopropanated molecules that, as Berkeley Lab explains, have “multiple triangle-shaped three-carbon rings that force each carbon-carbon bond into a sharp 60-degree angle. The potential energy in this strained bond translates into more energy for combustion than can be achieved with the larger ring structures or carbon-carbon chains typically found in fuels. In addition, these structures enable fuel molecules to pack tightly together in a small volume, increasing the mass – and therefore the total energy.”
How did we find them?
As they say in Australia, it was hard yakka, tough work. The team explains that they had been searching for “cyclopropane molecules for a long time. JBEI chief Jay Keasling had scoured the scientific literature for organic compounds with three-carbon rings and found just two known examples, both made by Streptomyces bacteria that are nearly impossible to grow in a lab environment. Fortunately, one of the molecules had been studied and genetically analyzed due to interest in its antifungal properties. Discovered in 1990, the natural product is named jawsamycin.”
From there, they’ve been working to engineer the organism to produce the molecules in relevant titers and yields.
“A lot of the drugs used today, such as immunosuppressants, antibiotics, and anti-cancer drugs, are made by engineered Streptomyces,” said lead author Pablo Cruz-Morales. “But they are very capricious and they’re not nice to work with in the lab. They’re talented, but they’re divas.” When two different engineered Streptomyces failed to make POP-FAMEs in sufficient quantities, he and his colleagues had to copy their newly arranged gene cluster into a more “tame” relative.
In the end, they’ve produced fatty acids with up to seven cyclopropane rings on a carbon chassis — once extra transesterification-like step converts the molecules to fuels.
The technical background
If you remember Syntin, a Soviet-era fuel that was used for the Soyuz program until they were abandoned over the health hazards from and soaring costs of by making them from petroleum, these molecules have performance and structural similarities — three cyclopropane rings, higher density, lower viscosity. The fuels are still discussed for their spectacular initial impulse, but the costs were at last glance something like $100/kilo, more than 10 times the cost of RP-1.
“The larger consortium behind this work, Co-Optima, was funded to think about not just recreating the same fuels from biobased feedstocks, but how we can make new fuels with better properties,” said Sundstrom. “The question that led to this is: ‘What kinds of interesting structures can biology make that petrochemistry can’t make?’”
Where are we in process development?
In the world of NASA technical readiness levels, right around TRL 4. As NASA explains, “Once the proof-of-concept technology is ready, the technology advances to TRL 4. n the world of biology, that means we know how to make it, now we need to increase the production efficiency even further to generate enough for combustion testing.
Plus, think about the opportunities in solid fuels.
“We’re working on tuning the chain length to target specific applications,” said Sundstrom. “Longer chain fuels would be solids, well-suited to certain rocket fuel applications, shorter chains might be better for jet fuel, and in the middle might be a diesel-alternative molecule.”
Then, removing the two oxygen molecules along for the ride is something tipped by the researchers. “Although POP-FAMEs share similar structures to Syntin, many have superior energy densities. Higher energy densities allow for lower fuel volumes, which in a rocket can allow for increased payloads and decreased overall emissions,” said author Alexander Landera, a staff scientist at Sandia. One of the team’s next goals to create a process to remove the two oxygen atoms on each molecule, which add dead weight. “When blended into a jet fuel, properly deoxygenated versions of POP-FAMEs may provide a similar benefit,” Landera added.
Down the line? Moving from sugar to waste. The researchers have tipped that going carbon negative is the ultimate goal, the Prize of Prizes.
Reaction from the stakeholders
“This biosynthetic pathway provides a clean route to highly energy-dense fuels that, prior to this work, could only be produced from petroleum using a highly toxic synthesis process,” said project leader Jay Keasling, a synthetic biology pioneer and CEO of the Department of Energy’s Joint BioEnergy Institute (JBEI). “As these fuels would be produced from bacteria fed with plant matter – which is made from carbon dioxide pulled from the atmosphere – burning them in engines will significantly reduce the amount of added greenhouse gas relative to any fuel generated from petroleum.”
To remove the oxygen, or not?
For missile fuel, by all means remove the oxygen, it’s dead weight given that the fuel can combust using atmospheric oxygen. But, maybe we should hold off de-oxygenating a rocket fuel. Out there in space, oxygen is not dead weight, in fact (like guys going to the summit of Everest) you have to carry it with you. The heaviest thing going to the Moon is the oxygen. So, when you have a high-performing molecule and some oxygen already in tow, perhaps leaving better alone is a good idea. Or, at least measuring the fuel efficiency in the full context of combustion in a vacuum. The presence of oxygen in ethanol is a primary reason it was admired by Werner von Braun as a rocket fuel.
The Bottom Line
We’d like to return to the theme we opened with. This is not just a new class of fuels. It is a Disturbance in the Force, and letting it out on the street is probably as wise as introducing Darth Vader to the ways of the Jedi. Even if one does not need the 25 percent increase in range, there’s the 25 percent in payload gain to consider as an alternative. Or the significant decrease in the radar cross-section.
Yes, these are Molecules for Mars, but maybe the other one.
Category: Top Stories















