E-fuels: Do they work on the numbers?
By Dr. Ashish Gupta, Process Manager, S&B; Muhammad Islam, Vice President, Commercial and Technology, S&B; and Clint Johnson, Vice President, Saber Equity
Special to The Digest
As the world works towards decarbonization, one sector contributing significantly to greenhouse gas (GHG) emissions is transportation. To address this issue, the concept of e-fuels has emerged as a promising solution. E-fuels, also known as synthetic fuels, are produced using renewable energy to power electrolysis of water into “green” hydrogen, which is then combined with carbon dioxide (CO2) to produce fuel (see Figure 1).
Figure 1: E-fuels Production Scheme
The International Maritime Organization (IMO) has set a target of reducing GHG emissions by 50% by 2050 compared to 2008 levels. This target has led to an increased focus on e-fuels, in particular, e-methanol.
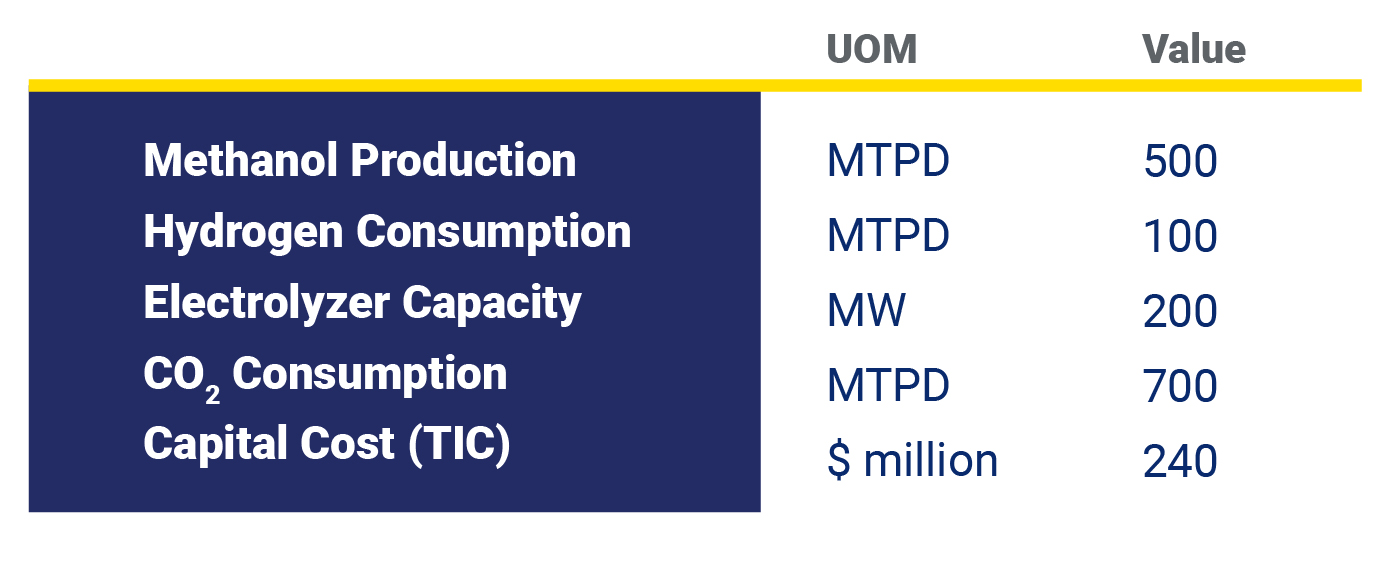
In this article, the economics of a hypothetical 500 metric ton per day (MTPD) e-methanol are explored. Our calculations assume the project purchases green hydrogen and CO2 from a third party. However, the findings are still valid if a project produces its own green hydrogen or even produces its own renewable power. Table 1 summarizes the key project assumptions considered.
Table 1 – Project Summary
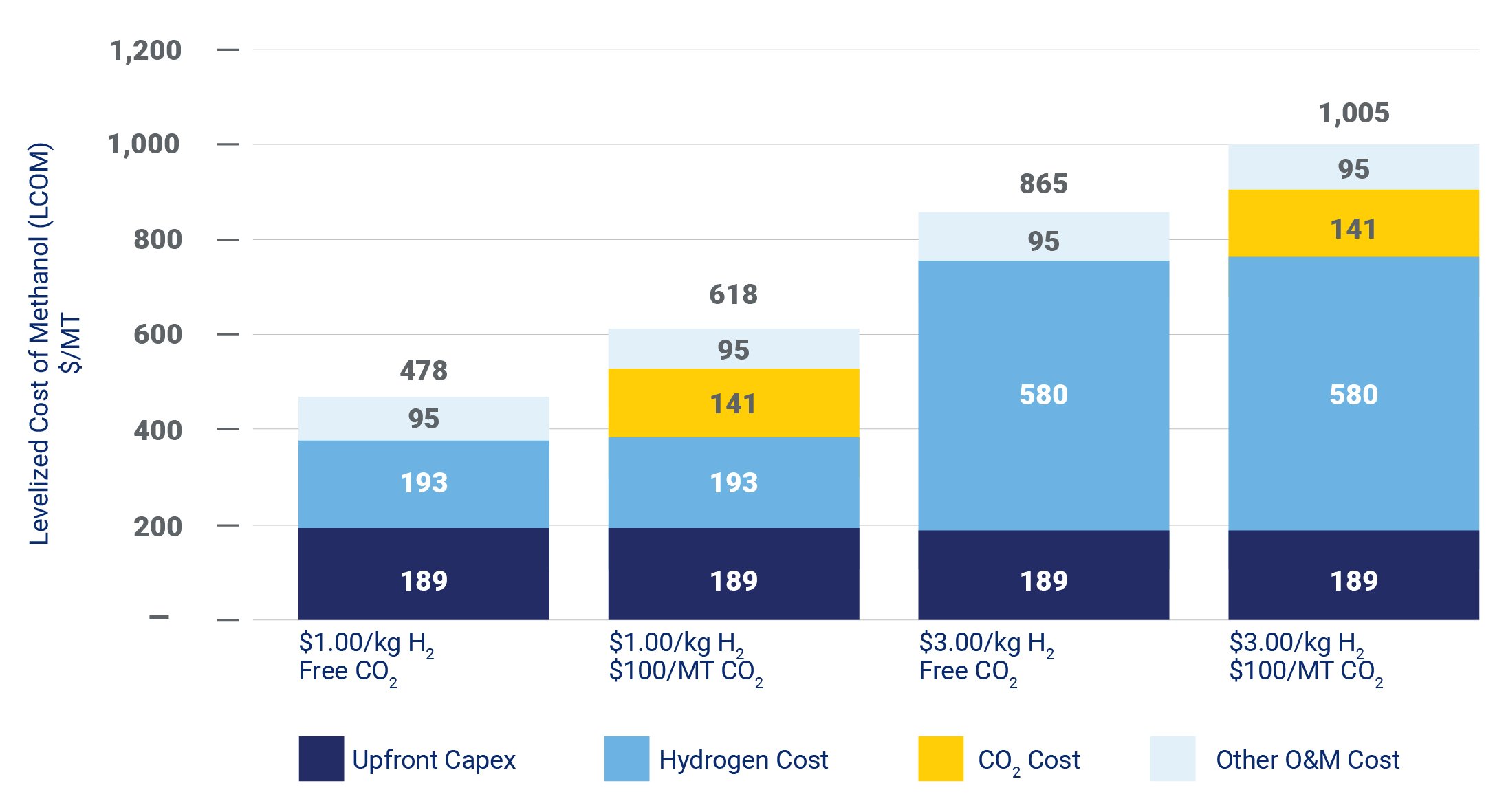
Hydrogen cost is the most significant driver of the cost of e-methanol. Each additional $1 per kilogram of hydrogen price adds almost $200 per metric ton (MT) to the levelized cost of methanol. The economics of e-methanol—like so many other low-carbon products—are therefore heavily dependent on driving down the cost of green hydrogen.
Although less significant than hydrogen, the cost of CO2 is also an important economic driver. Each additional $50/MT of CO2 price adds approximately $70/MT to the levelized cost of methanol.
Figure 2 – Levelized Cost of e-Methanol
Another factor to consider is the carbon intensity (CI) of the fuel. CI is the amount of GHG emissions associated with producing and using a fuel, typically expressed in grams of CO2 equivalent (CO2e) per megajoule (MJ) of energy content.
Traditional “gray” methanol has CI of about 100 grams of CO2e per MJ, compared to about 93 for typical maritime fuel—very low sulfur fuel oil (VLSFO). This means that producing and burning gray methanol to fuel ships generates about 10% more GHG emissions than VLSFO.
Figure 3 – Carbon Intensity of Maritime Fuels
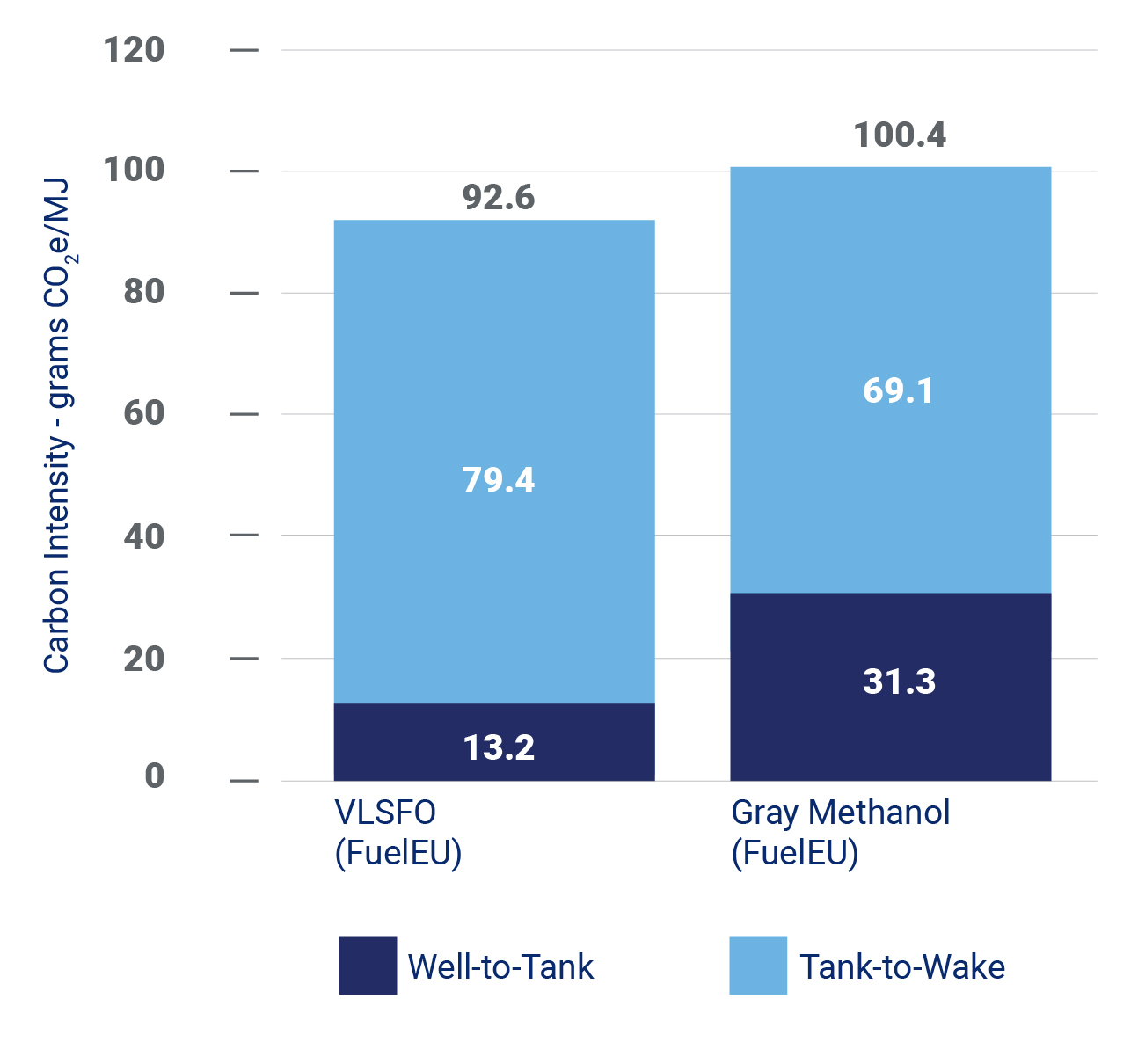
E-methanol, however, can potentially have CI as low as about 69 CO2e per MJ—which is just the emissions associated with burning the fuel. The actual CI will depend on the power source, transportation distance, and other factors. This suggests e-methanol could reduce GHG emissions in maritime fuels by over 25%, compared to traditional VLSFO.
European Union (EU) policies are being implemented that establish a price for the carbon intensity of maritime fuels. This is creating a premium price for low-carbon methanol in the EU, as shippers can afford to pay more for fuel that will save them money on tax penalties. These policies have spurred a recent uptick in e-methanol development activity.
The e-methanol market is relatively new and non-transparent. However, some published sources indicate market prices for e-methanol in excess of $1,000/MT. The source of the CO2 is a critical consideration in evaluating market pricing, and some of the highest premiums may only be available for e-methanol produced from “biogenic” CO2. In any case, sufficient price premiums may indeed be available to make e-methanol economically viable, depending on the price of hydrogen and CO2.
In addition to securing attractive prices for the feedstocks and product, the success of any project will depend on partnering with a strong engineering, procurement, and construction (EPC) provider that understand what it takes to deliver a successful project.
Category: Thought Leadership, Top Stories















